SODIUM HYDROXIDE
- Home
- urunler
- diğer kimyasallar
- KİMYASALLAR
- SODIUM HYDROXIDE
OTHER CHEMICALS
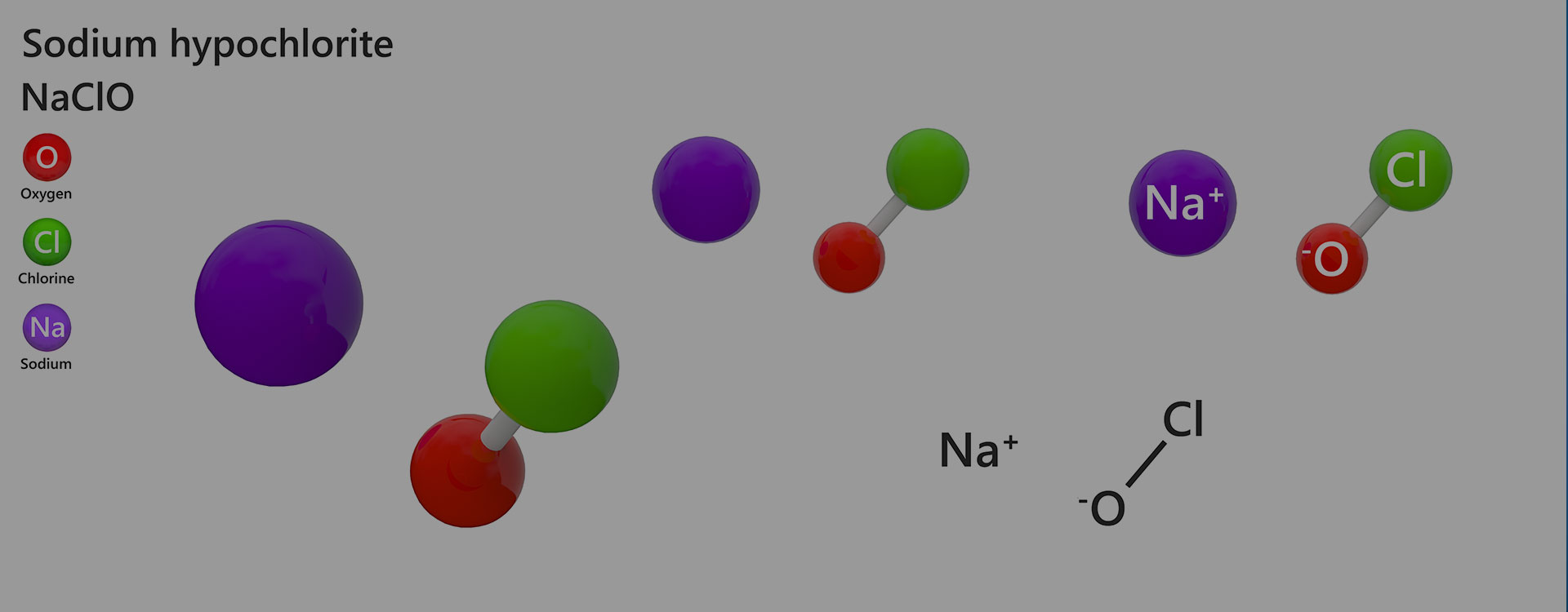
SODIUM HYPOCHLORITE
Sodium hypochlorite is a type of salt. It is used in bleaching bleaches in daily life. It is produced by reacting chlorine at room conditions with sodium hydroxide in soaps. The formula for obtaining sodium hypochlorite is as follows: 2NaOH + Cl₂ → NaCl + NaClO + H₂O
- Formula: NaClO
- Density: 1.11 g/cm³
- Molar mass: 74.44 g/mol
- Melting point: 18 °C
- Boiling point: 101 °C
- Substance dissolved in: Water
Share on facebook
Share on twitter
Share on linkedin
TDS
OTHER CHEMICALS
To order this product, proceed from the order registration link or contact us at the contact numbers.
FOR ORDER| Physical and Chemical Properties | ISIRI Compliant Range | CCPC Product Range | Standard Reference |
| Purity of Sodium Hydroxide (NaOH) _ %Mass | Min 98 | Min 98 | ISIRI 364 |
| Carbonate as Na2CO3 _ %Mass | Max 1 | Max 0.6 | ISIRI 364 |
| Chloride as NaCl _ %Mass | Max 0.06 | Max 0.02 | ISIRI 364 |
| Sulfate as Na2SO4 _ %Mass | Max 0.01 | Max 0.005 | ISIRI 364 |
| Silicate as SiO2 _ %Mass | Max 0.02 | Max 0.005 | ISIRI 364 |
| Iron as Fe _ mg/Kg | Max 30 | Max 15 | ISIRI 364 |
| Insoluble in Water _ %Mass | Max 0.1 | 0 | ISIRI 364 |
| Aluminium as Al2O3 mg/Kg | Max 20 | Max 20 | ISIRI 364 |
| Heavy Metals as Pb mg/Kg | Max 20 | Max 20 | ISIRI 364 |
| Mercury as Hg _ mg/Kg | Max 0.2 | < 0.15 | ISIRI 364 |
| Arsenic as As_mg/Kg | Max 2 | Max 0.05 | ISIRI 364 |
| Appearance | White Color, Free of Visible Impurities | OK | ISIRI 364 |